Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8 - OXIDATION/PHOSPHORYLATION
C: ATP AND OXIDATIVE PHOSPHORYLATION
BIOCHEMISTRY - DR. JAKUBOWSKI
04/15/16
|
Learning Goals/Objectives for Chapter 8C: After class and this reading, students will be able to
|
C2. Anerobic Coupling of Oxidation and ATP Synthesis
Our main goal is to understand how oxidation reactions can lead to ATP synthesis. First let us consider ATP production under anaerobic condition, such as which often occurs during the fight or flight response. You know how terribly you feel when you run a 100 m dash. Your muscles feel horribly due to lactic acid buildup, and you know you can't seem to get enough dioxygen into your body. Under these conditions, a pathway called glycolysis (which you studied in biology) is active. In this pathway, glucose, a 6 carbon hexose, is converted to two, 3C molecules - pyruvate. A detail description is shown below.
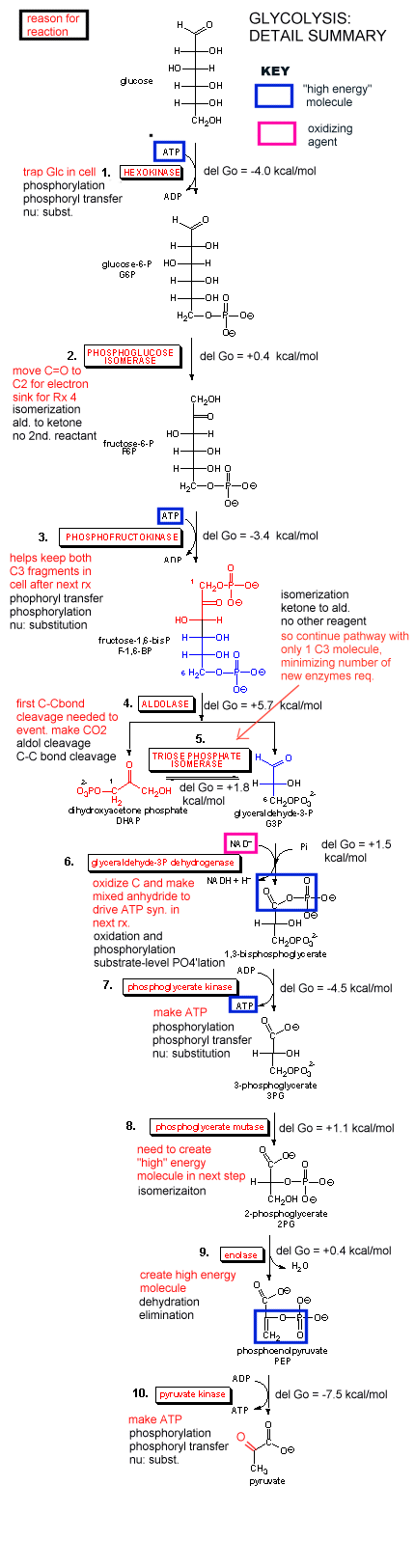
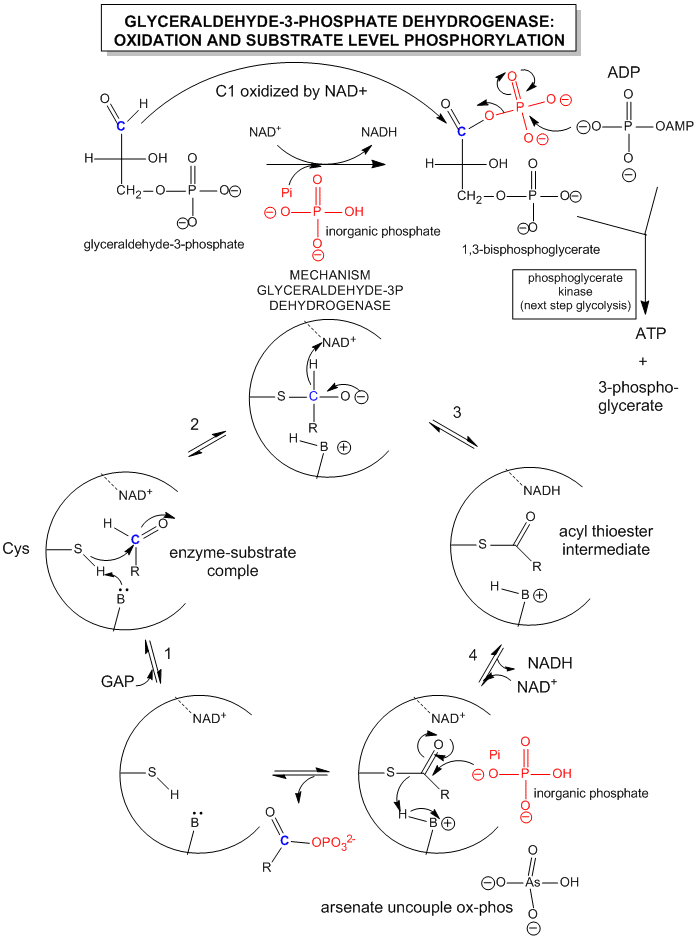
Since we most interested in energy transduction at this point, let's consider just two important step in glycolysis that directly lead to ATP synthesis. Only one oxidative step is found in this pathway, namely the oxidative phosphorylation of the 3C glycolytic intermediate glyceraldehyde-3-phosphate, to 1,3-bisphosphglyercate, a mixed anhydride (see link below for mechanism). The oxidizing agent is NAD+ and the phosphorylating agent is NOT ATP but rather Pi. The enzyme is named glyceraldehyde-3-phosphate dehydrogenase. It contains an active site Cys, which helps explain how the enzyme can be inactivated with a stoichiometric amounts of iodoacetamide. A general base in the enzyme abstracts an H+ from Cys, which attacks the carbonyl C of the glyceraldehyde, forming a tetrahedral intermediate. Instead of the expected reaction (which would be the protonation of the alkoxide in an overall nucleophilic addition reaction at the aldehyde), a hydride leaves from the former carbonyl C to NAD+ in an oxidation step. Notice, this is a two electron oxidation reaction similar to seen in alcohol dehydrogenase. An acyl-thioester intermediate has formed, much like the acyl intermediate that formed in Ser proteases. Next inorganic phosphorous, Pi, attacks the carbonyl C of the intermediate in a nucleophilic substitution reaction to form the mixed anhydride product, 1,3-bisphoshphoglycerate. Although we have formed a mixed anhydride, we cleaved a sulfur ester, which is destabilized with respect to its hydrolysis products (since the reactant, the thioester, is not stabilized by resonance to the extent of regular esters owing to the poor donation of electrons from the larger S to the carbonyl-like C.) In the next step, catalyzed by the enzyme phosphoglycerate kinase, ADP acts an a nucleophile which attacks the mixed anhydride of the 1,3-bisphosphoglyerate to form ATP. Note that the enzyme is name for the reverse reaction. We have coupled oxidation of an organic molecule (glyceraldehyde-3-phosphate) to phosphorylation of ADP through the formation of a "high" energy mixed anhydride, 1,3-bisphosphoglycerate.
The linkage between oxidation of glyceraldehyde-3-phosphate and the phosphorylation of ADP by 1,3-bisphosphoglycerate can be artificially uncoupled by adding arsenate, which has a similar structure as phosphate. The arsenate can form a mixed anhydride at C1 of glyceraldehyde-3-phosphate, but since the bridging O-As bond is longer and not as strong as in the mixed anhydride, it is easily hydrolyzed. This prevents subsequent transfer of phosphate to ADP to form ATP.
![]() Jmol:
Updated Glyceraldehyde-3-phosphate dehydrogenase (NAD)
Jmol14 (Java) |
JSMol (HTML5)
Jmol:
Updated Glyceraldehyde-3-phosphate dehydrogenase (NAD)
Jmol14 (Java) |
JSMol (HTML5)
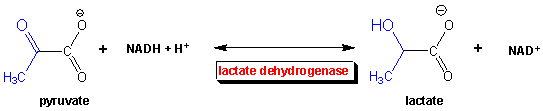
Under anaerobic conditions, glucose is metabolized through glycolysis which converts it to two molecules of pyruvate. Only one oxidation step has been performed when glyceraldehyde 3-phospate is oxidized to 1,3-bisphosphoglycerate. To regenerate NAD+ so glycolysis can continue, pyruvate is reduced to lactate, catalyzed by lactate dehydrogenae. These reactions take place in the cytoplasm of cells actively engaged in anaerobic oxidation of glucose (muscle cells for examples during sprints). Note that the enzyme is named for the reverse reaction, the oxidation of lactate by NAD+.
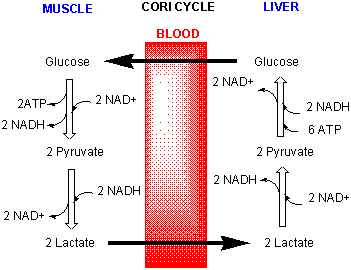
Lactate in the muscle can go by way of the blood to the liver (where NAD+ is not depleted) and be converted back to pyruvate and eventually back to glucose through a pathway called gluconeogenisis. The liver can export the glucose into the blood from where it can be taken up by the muscle for ATP production. This cycle is called the Cori cycle.
Figure: Cori Cycle
![]() Moodle
Online Quiz (PASSWORD PROTECTED): GLYCOLYSIS
Moodle
Online Quiz (PASSWORD PROTECTED): GLYCOLYSIS
Navigation
Return to Chapter 8C: ATP and Oxidative Phosphorylation
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8C: ATP and Oxidative Phosphorylation

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.