Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8 - OXIDATION/PHOSPHORYLATION
C: ATP AND OXIDATIVE PHOSPHORYLATION
BIOCHEMISTRY - DR. JAKUBOWSKI
04/15/16
|
Learning Goals/Objectives for Chapter 8C: After class and this reading, students will be able to
|
C4. An Overview of Mitochondrial Electron Transport
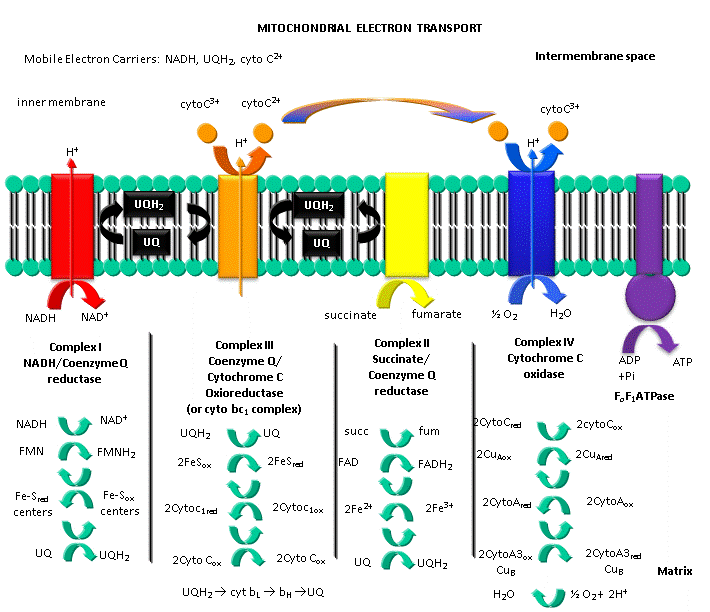
The main oxidizing agent used during aerobic metabolism is NAD+ (although FAD is used in one step) which get converted to NADH. Unless the NAD+ can be regenerated, glycolysis and the Kreb's cycle will grind to a halt. Luckily, under these conditions we are actually continually breathing one of the best oxidizing agents around, dioxygen. NADH is oxidized back to NAD+ not directly by dioxygen, but indirectly as electrons flow from NADH through a series of electron carriers to dioxygen, which gets reduced to water. This process is called electron transport. No atoms of oxygen are incorporated into NADH or any intermediary electron carrier. Hence the enzymes involved in the terminal electron transport step, in which electrons pass to dioxygen, is an oxidase. The enzymes of the Kreb's cycle and electron transport are localized in mitochondria.
Figure: mitochondria
By analogy to the coupling mechanism under anaerobic conditions, it would be useful from a biological perspective if this electron transport from NADH to dioxygen, a thermodynamically favorable reaction (as you calculated in the last study guide - a value of about -55 kcal/mol), were coupled to ATP synthesis. It is! For years scientist tried to find a high energy phosphorylated intermediate, similar to that formed by glyceraldehyde-3-phosphate dehydrogenase in glycolysis, which could drive ATP synthesis (which likewise occurs in the mitochondria). None could be found. A startling hypothesis was put forward by Peter Mitchell, which was proven correct and for which he was awarded the Nobel Prize in Chemistry in 1978. The immediate source of energy to drive ATP synthesis was shown to come not from a phosphorylated intermediate, but a proton gradient across the mitochondrial inner membrane. All the enzymes complexes in electron transport are in the inner membrane of the mitochondria, as opposed to the cytoplasmic enzymes of glycolysis. A pH gradient is formed across the inner membrane occurs in respiring mitochondria. In electron transport, electrons are passed from mobile electron carriers through membrane complexes back to another mobile carrier. Initially, NADH shuttles electrons (2 electron oxidation, characteristic of NAD+/NADH), to a flavin derivative, FMN, covalently attached to Complex I. The reduced form of FMN then passes electrons in single electron steps (characteristic of FAD-like molecules, which can undergo 1 or 2 electrons transfers) through the complex to the lipophilic electron carrier, ubiquinone, UQ.
Figure: lipophilic electron carrier, ubiquinone, UQ
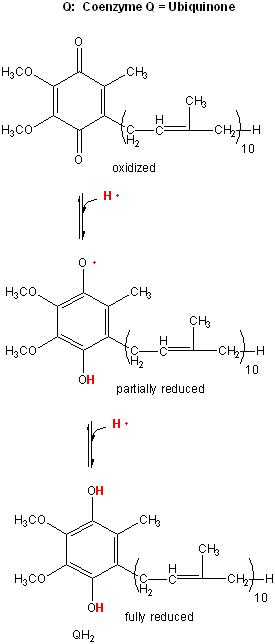
This then passes electrons through Complex III to another mobile electron carrier, a small protein, cytochrome C. Then cytochrome C passes electrons through complex IV, cytochrome C oxidase, to dioxygen to form water. At each step electrons are passed to better and better oxidizing agents, as reflected in their increasing positive standard reduction potential. Hence the oxidation at each complex is thermodynamically favored.
![]() Jmol:
Updated Cytochrome C Oxidase
Jmol14 (Java) |
JSMol (HTML5)
Jmol:
Updated Cytochrome C Oxidase
Jmol14 (Java) |
JSMol (HTML5)
Complex II (also called succinate:quinone oxidoreductase) is a Kreb cycle enzyme that catalyzes the oxidation of succinate to fumarate by bound FAD (hence its other name: succinate dehydrogenase). It is not involved in flow of electrons from NADH to dioxygen described above but passes electrons from the reduced succinate to ubiquinone to form fumarate and reduced ubiquone which then can transfer electrons to cytochrome C through Complex III. The crystal structure of this complex has recently been solved by Yankovskaya et al. who have shown that the arrangement of the redox-active sites in the complex minimizes potential oxidation of bound FADH2 by dioxygen, minimizing production of harmful reactive oxygen species like superoxide.
![]() Animation
of electron transport in mitochondria
Animation
of electron transport in mitochondria
![]() Jmol:
Updated Succinate Dehydrogenase (Complex II)
Jmol14 (Java) |
JSMol (HTML5)
Jmol:
Updated Succinate Dehydrogenase (Complex II)
Jmol14 (Java) |
JSMol (HTML5)
At each complex, the energy released by the oxidative event is used to drive protons through each complex from the matrix to the intermembrane space of the mitochondria, and is not used to form a high energy mixed anhydride as we saw in the glyceraldehyde-3-phosphate dehydrogenase reaction. The actual mechanism of proton transfer is unclear.
Figure: ELECTRON TRANSPORT AND PROTON GRADIENT FORMATION IN THE MITOCHONDRIA
Navigation
Return to Chapter 8C: ATP and Oxidative Phosphorylation
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8C: ATP and Oxidative Phosphorylation

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.