Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 9 - SIGNAL TRANSDUCTION
B: NEURAL SIGNALING
BIOCHEMISTRY - DR. JAKUBOWSKI
06/10/14
|
Learning Goals/Objectives for Chapter 9B:
|
B1. Introduction to Neural Signaling
Neurochemistry is one of the most explosive areas of biological research. Scientists are now starting to unravel the molecular bases for memory, cognition, emotion, and behavior. The next decades will bring truly revolutionary understanding of brain chemistry and along with it the potential to alter human mood, memory, and to treat mental illness such as schizophrenia much more effectively. The human brain, with about 86 billion neurons. Compare this with some estimates for the number of stars in the Milky Way galaxy (about 100 billion, a number derived from luminosity and mass measurements). Now imagine that each neuron can form connections - synapses - with 1000 to 10,000 other neurons. Throw in another 86 billion brain glial cells and you have one of the most complex structures in the universe. This section will explore the biology and chemistry of neurons.
We will discuss two kinds of neurons - those that interact with muscles at the neuromuscular junction and those that interact with other neurons in the central nervous system. Neurons consist of a single, nucleated cell body with multiple signal-receiving outgrowths (dendrites) and multiple-signal sending outgrowths (axons) which end in a terminal button. These interact through the synapse with dendrites on other neurons.
Figure: Neurons
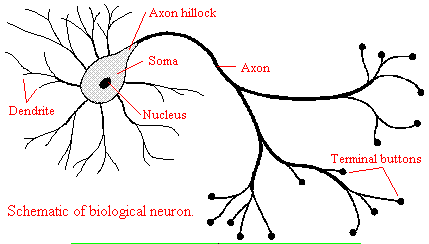
A presynaptic neuron can stimulate an adjacent postsynaptic neuron by releasing a neurotransmitter into the synapse between the cells, which binds to a receptor in the membrane of the post-synapatic cell, stimulating the cell.
Figure: neurotransmitter into the synapse between the cells
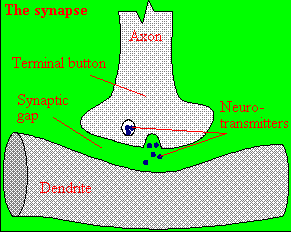
We will discuss the events which cause the post-synaptic cell to "fire", but we will not discuss the immediate events which lead to the release of neurotransmitter by the presynaptic neuron.
Neurons (as do all cells) have a transmembrane potential across the membrane. Transient changes in the membrane potential are associated with neuron activation or inhibition. This arises in part due to the imbalance of ions across the membrane which was established by the Na+-K+-ATPase in the membrane. This electrogenic antiporter transfers 3 Na ions out of the cytoplasm for every 2 K ions it transports in. Likewise Cl- has a much higher level outside the cell. Membrane potentials are determined not only by the size of the ion gradients across the membrane, but also the differential permeability of membranes to ions. As we saw previously, synthetic bilayer membranes are not very permeable to ions .
Ion permeability of phosphatidyl serine vesicles
| ION | PERMEABILITY (cm/s) |
| sodium | <1.6 x 10-13 (lowest) |
| potassium | <9 x 10-13 |
| chloride | <1.5 x 10-11 (highest) |
Sodium would be expected to have a lower permeability than potassium since it has a higher charge density. It also is largest in effective size due to the larger hydration sphere around the ion arising from its higher charge density. Chloride would be expected to have the highest permeability since it has the lowest charge density (due to repulsion of the electron cloud in the negative ion). Intracellular charged proteins (which are mostly negative) are not permeable and help in creating the negative charge imbalance across the membrane.
Much work has been done on the giant axon of the squid, which has uniquely high intracellular potassium (400 mM compared to 20 mM outside) and high extracellular fluid sodium (440 mM vs, 50 mM inside) and chloride (560 mM vs 52 mM inside). Mammalian cell concentrations are much lower, but the relative size of the gradient is about the same.
Typical ion concentrations and permeabilities for mammalian membranes.
| Ion | Cell (mM) | Blood (mM) | Permeability (cm/s) |
| potassium | 140 | 5 | 5 x 10-7 |
| sodium | 5-15 | 145 | 5 x10-9 |
| chloride | 4 | 110 | 1 x 10-8 |
| X- (neg. macromol.) | 138 | 9 | 0 |
How can we account for the markedly greater permeabilities of ions (1000x to 1,000,000 x) in mammalian cell membranes compared to synthetic lipid vesicles? Previously, we showed that glucose has a greater permeability through red blood cell membranes than through synthetic liposomes because of a membrane receptor that allows facilitated diffusion across the membrane and down a concentration gradient. The same thing is true of ion permeabilites in intact biologicial membranes. These membranes have several types of selective ion channels (nongated - always open, and gated - open only after specific conformational changes). The nongated channels dramatically increase the permeability of membranes to ions, as the glucose transport protein increased the permeabilty to glucose. It turns out that this differential permeability contributes to the transmembrane potential. Ion channels in nerve and muscle can move ions across the membrane at a rate up to 109/s, which is comparable to kcat for the best enzymes.
If we envision channels as pores, how can we account for the selectivity of the channel to specific ions. A larger pore should admit any ion less than a maximal size for the pore, so it is hard to image the nature of the selectivity filter. Because of this, many people discounted the ideas of channels in favor of a transporter, which would bind the ion selectivity and then, through conformational changes, move the ion across (much like the Na/K ATPase we discussed in the previous guide). This model could not, however, account for the incredible rates of ion flux across the membrane. Selectivity can be accounted for by a channel that contains a narrow opening that acts as an ion sieve. The ion looses most of its hydration sphere and would form specific interactions with amino acid side chains in the pore region. Such an interaction would be transient and not too tight since the ion must pass through the membrane. As we will see later, these ion channels:
- pass ions down a concentration gradient in a thermodynamically favorable process
- are specific for certain ions (although a few are less selective and will pass Na, K, Ca, and Mg ions)
- allow ion flow through either ungated or gated channels.,
- saturate with increasing ion concentration (even though as concentration increases, the ions have a greater thermodynamic drive to pass through the channel). This is consistent with binding of the ion at a selectivity filter in the narrow part of the pore. The Kd for the interaction is usually in the mM range and indicates weak binding with large dissociation rate constants (koff).
Navigation
Return to Chapter 9B: Neural Signaling Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 9B: Neural Signaling

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.