Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 9 - SIGNAL TRANSDUCTION
C: SIGNALING PROTEINS
BIOCHEMISTRY - DR. JAKUBOWSKI
04/16/16
|
Learning Goals/Objectives for Chapter 9C:
|
Estonian Translation √ by Anna Galovich
C6. G Protein Coupled Receptors (GPCR) and G proteins
Receptors that interact with G proteins (G protein coupled receptors or GPCRs) have common characteristics. GPCRs are single polypeptides which have 7 membrane-spanning α- helices. Over 800 similar GPCR receptor genes are found in humans, each encoding a protein of similar topology but which bind different ligands. Many of the receptors bind to unknown ligands, and hence are called orphan receptors.
The β-adrenergic receptor is a prototypical GPCR. Found in muscle, liver, and fat cells, it bind epinephrine and adrenaline which leads to energy mobilization and muscle activation (i.e. flight or fight response). The mechanism of activation of a GPCR is illustrated using the beta adrenergic receptor as an example. The unoccupied adrenergic receptor is associated with a heterotrimeric G protein, which contains an α, β,andγsubunits. GDP is usually bound to the αsubunit. When the hormone is bound to the receptor, interactions of the receptor with the G protein (probably through the βandγsubunits leads to conformational changes in the G protein leading to replacement of GDP with GTP. This promotes dissociation of the αsubunit (with bound GTP), which is then free to bind modulate the activity of an adjacent membrane protein, adenylate cyclase. The αsubunit is held to the membrane through a lipid anchor attached through a post-translational modification. As long as GTP remains bound to the Gα subunit, it will continue to modulate the activity of adenylate cyclase. A built in regulatory mechanism does exist in the protein since the Gα subunit has GTPase activity. The GTP will eventually hydrolyze, the GDP-Gα subunit will lose affinity for its bound partner (adenylated cyclase), and return to the heterotrimeric G protein associated with the unbound receptor.
GPCRs appear to bind ligand in a binding cavity localized at the extracellular face and between four of the transmembrane helices. Upon binding of a natural ligand, a conformational change in the arrangement of the helices occurs, allowing access of the Gα subunit to the GPCR. In the ternary complex of GPCR:Ligand:G protein, the affinity of the GPCR for the agonist and of the Gα subunit for GTP (over GDP) are increased.
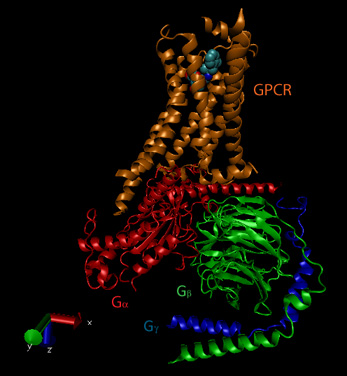
The activity and structure of GPCRs have been studied using natural ligands (hormones and neurotransmitters), as well as agonists, partial agonists, inverse agonists and antagonists. As discussed previously, agonists bind to the natural ligand binding site and elicit the same or a partial response (partial agonist). Inverse agonists bind and lower the response of the constituitively active receptor, and antagonists bind and prevent the normal response of an agonist. The figure below shows a crystal structural of the beta-andrenergic:Gs complex with bound agonist.
Figure: Beta-andrenergic:Gs complex (Image made with VMD)
![]() Updated
Beta andrenergic receptor
Jmol14 (Java) |
JSMol (HTML5)
Updated
Beta andrenergic receptor
Jmol14 (Java) |
JSMol (HTML5)
![]() Updated
Beta andrenergic receptor:Gs Complex
Jmol14 (Java) |
JSMol (HTML5)
Updated
Beta andrenergic receptor:Gs Complex
Jmol14 (Java) |
JSMol (HTML5)
![]() Updated
Models of the GPCR- Melancortin 4 Receptor
Jmol14 (Java) |
JSMol (HTML5)
Updated
Models of the GPCR- Melancortin 4 Receptor
Jmol14 (Java) |
JSMol (HTML5)
Some bacterial toxins work by inactivating the GTPase activity of the Gα subunit, keeping it in the "stuck" position. For example, cholera toxin, an enzyme released by Vibrio cholerae , catalyzes the ADP ribosylation of an Arg in the Gα subunit by transferring everything but the nicotinamide from NAD+ to the Arg residue.
In contrast to the beta-adrenergic receptor, some Gα subunits actually inhibit adenylate cyclase when bound. These Gα subunits are called Gai/o in contrast to the stimulatory subunits, Gas. Gα subunits interact with proteins other than adenylate cyclase. We have already seen an example with the PKC activation of phospholipase C which is activated by the Gaq11 . There are many different Gα-like subunits expressed in different tissues. Four structural classes and at least twenty variants of Ga have been found.
Examples of different signals, receptors, Gα like-subunits, second messenger changes, and affected intracellular enzymes
| signal | vasopressin | epinephrine | light | odorant | odorant | sweet tastant |
| receptor | VR | β-adrenergic | rhodopsin | odorant recep. 1 | odorant recept. 2 | sweet receptor |
| Ga like- subunit | Gi | Gs | transducin | Golfactory | Golfactory | Ggustatory |
| coupled enzyme | adenylate cyclase | adenylate cyclase | phosphodiesterase | phopholipase C | adenylate cyclase | adenylate cyclase |
| 2nd messenger | decrease cAMP | increase cAMP | decrease cGMP | increase IP3 | increase cAMP | increase cAMP |
| protein affected | decrease PrK-A | increase PrK-A | dec. Ca, Na perm. | inc. Ca perm | inc.Ca, Na perm | dec. K perm |
Another variant of a GDP/GTP binding protein is ras. Mammalian cells contain 3 variants of ras: H, K, and N. They all bind GDP/GTP and have GTPase activity, and are members of a large family of small GTPase proteins. This protein is targeted to the cell membrane through the post-translational addition of a hydrophobic farnesyl group. When activated by binding to GTP, it can to and activated a protein call Raf-1, which is activated to become a tyrosine kinase.
Changeux and Edelstein reviewed the MHC model 40 years after its conception and support its application to signal transduction processes. They include in siganling molecules not only hemoglobin, but regulatory enyzmes (aspartate transcarbamylase, phosphofructokinase, LDH, glycogen phosphorylase), membrane receptors (acetylcholine receptor, rhodopsin), and nuclear receptors (lac repressor, steroid hormone receptors). In all these signaling proteins, residue distant from the "active" site participate in binding to allosteric ligands. Often the allosteric site is on a separate domain which can be cleaved from the protein and still maintain allosteric ligand binding properties. The proteins also consists of multiple subunits easily related by distinct symmetry axes. Allosteric ligands often bind in cavity in subunit interfaces along symmetry axes. In general, crystal structure analyses show that low affinity T and high affinity R forms of the signaling proteins exists, but accompanied by minor tertiary structure changes in individual subunits (i.e. perfect symmetry in all subunits is not preserved on binding of allosteric ligand). For neurotransmitter membrane receptors, these two states can be correlated with an open and closed state (for ion flux), and open conformations of these proteins can often be found in mutant forms. However, for many ligand-gated ion channels and G-protein coupled receptors (serpentine), kinetic analyses show more complicated forms than can be represented by a simple two state (R and T) model. High-resolution microscopy shows evidence for nonsymmetrical quaternary structural changes. These change can be observed in the absence of ligand, which gives support to the MWC concept that allosteric ligands select certain conformational states, leading to equilibrium shifts in the unliganded receptor to the more high affinity state. More refined methods of structural analysis will presumably show more evidence of subtle tertiary changes in the proteins that are preludes to quaternary structural changes. Yet the simplicity of the MWC model for explaining many features of signaling proteins remains.
Navigation
Return to Chapter 9C. Signaling Proteins Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 9C: Signaling Proteins

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.